

To treat calcific aortic stenosis, Cardiawave has developed a real-time image-guided non-invasive ultrasound therapy medical device using a cutting-edge ultrasound technology. Non-Invasive Ultrasound Therapy (NIUT) consists in repairing the aortic valve, not replacing it. No surgery is required. Our unique approach could represent both an alternative and a complementary approach to current procedures.
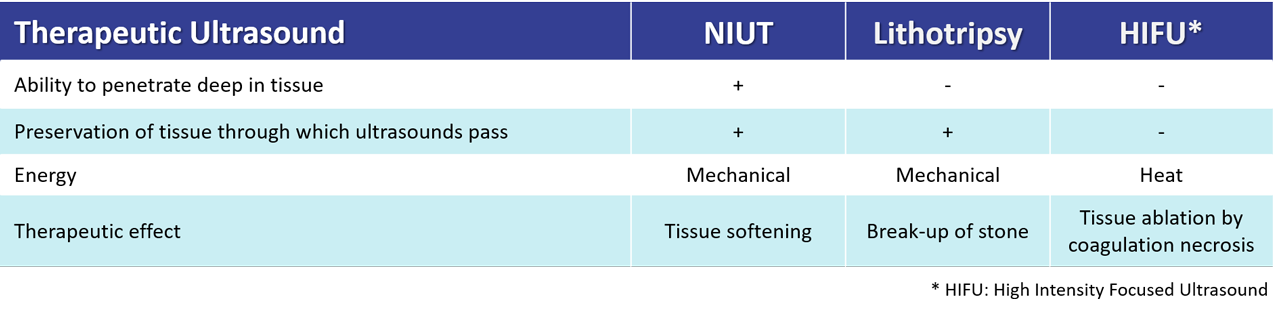
Valvosoft®, delivers focused high-intensity ultrasounds with precision to perform a remote and reparative effect on the aortic valve leaflets. The energy is mechanical (bubbles cavitation and shockwaves), non-thermal. Aortic valve leaflets tissues are softened, restoring their mobility and improving the anatomical and hemodynamical parameters following the procedure.
The disruptive and patented technology developed by Cardiawave is unique and will offer several advantages over state-of-the-art. It will allow physicians to deliver with a non-invasive ultrasound therapy medical device mechanical energy on a moving target with precision, while at the same time preserving the tissue and organs through which the ultrasound passes. Unlike any existing technology, it is possible to target and treat, several anatomical zones spread over a large area without physically moving the medical device, which is placed on the patient’s chest, over the beating heart. Treatment is monitored by the user via ultrasound imaging in real time.

Patients severely affected and not eligible to current medical response should benefit from our treatment.

Our treatment is completely non-invasive. No surgery is required. We expect the procedure to rapidly become ambulatory.

Our solution should improve the quality of life of patients.

Our investigational device should decrease mortality, risks and complications.

Our treatment may be repeated.

Our investigational device could slow down the progression of the disease and be used as preventive treatment for young patients or at an earlier stage of the disease.